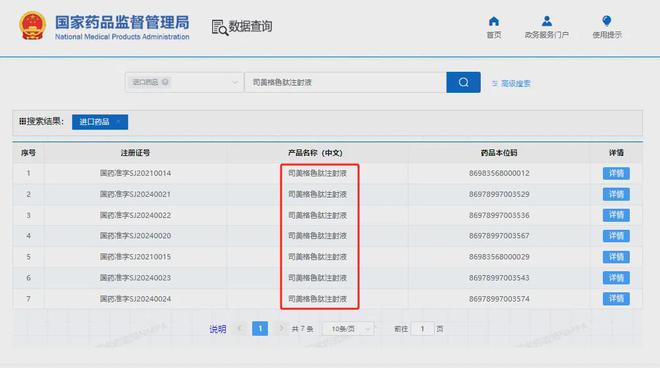
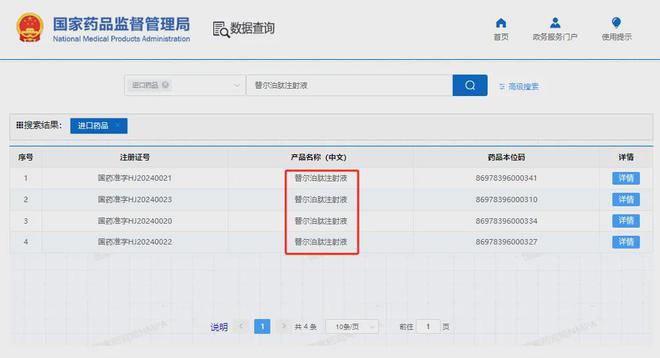
On January 2, 2025, Eli Lilly announced that its self-developed GIP/GLP-1 receptor dual agonist tirzepatide injection, Mounjaro, officially launched in China. Mounjaro covers two indications: "type 2 diabetes" and "weight loss," which were approved by the National Medical Products Administration in May and July 2024,respectively.

It is reported that there are four specifications of Mounjaro launched in China, including 2.5mg:0.5ml, 5mg:0.5ml, 7.5mg:0.5ml, and 10mg:0.5ml. At the same time, Mounjaro is already available on e-commerce platforms such as JD Health and Meituan for tirzepatide diagnosis and medication services, with varying prices among different merchants. Tirzepatide is a GLP-1/GIP receptor dual agonist developed by Eli Lilly, which can produce synergistic effects on appetite, calorie intake, and metabolic function. Both GLP-1 and GIP are incretins, which are peptides secreted by the gastrointestinal mucosa. GLP-1 can bind to receptors on pancreatic islet cells and affect insulin secretion, thereby producing a hypoglycemic effect, and can also delay gastric emptying and control appetite, thus maintaining weight; GIP has effects such as inhibiting gastric acid and pepsin secretion, affecting insulin release, and controlling gastrointestinal motility and emptying, filling the gap in the action of GLP-1 receptor agonists. Abroad, Eli Lilly's "hypoglycemic injection version" of tirzepatide was approved by the US FDA in May 2022, with the brand name Mounjaro; the "weight loss injection version" was approved by the US FDA in November 2023, with the brand name Zepbound. In China, Eli Lilly's "hypoglycemic injection version" of tirzepatide was approved by the National Medical Products Administration on May 21, 2024, with the brand name Mounjaro; the "weight loss injection version" was approved on July 19, 2024, also with the brand name Mounjaro. In addition to weight loss and diabetes, Eli Lilly is expanding the range of indications for tirzepatide, with a total of 9 chronic disease indications laid out for cardiovascular diseases, chronic kidney disease, NASH, etc.

About Eli Lilly

Eli Lilly, founded in 1876 and headquartered in Indianapolis, Indiana, USA, is a globally renowned pharmaceutical company with a long history. It conducts drug clinical trials in more than 50 countries worldwide and has research and development centers in 8 countries, with pharmaceutical production enterprises in 13 countries, and its products are marketed in 143 countries and regions.

Eli Lilly's business scope covers multiple fields, including diabetes, the nervous system, oncology, etc. The core business is the diabetes business, which mainly includes insulin products and oral hypoglycemic drugs. Among them, insulin products are the main contributors to this business, with a variety of different types of insulin, such as long-acting insulin, short-acting insulin, premixed insulin, etc.

In the field of endocrinology, Eli Lilly and Novo Nordisk hold a leading position in the insulin market. The approval year of tirzepatide is later than that of semaglutide. Semaglutide is a GLP-1 receptor agonist developed by Novo Nordisk, which has been approved for the control of type 2 diabetes and weight loss. It mainly reduces appetite and thus reduces calorie intake by changing the signals of hunger and satiety in specific neural areas.
Tirzepatide is a dual agonist of the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor developed by Eli Lilly, which can produce synergistic effects on appetite, calorie intake, and metabolic function. So far, Eli Lilly's tirzepatide Mounjaro has been approved for diabetes and weight loss indications in China.
On April 28, 2022, Eli Lilly announced the phase 3 clinical data of SURMOUNT-1, targeting 2539 overweight or obese adults without diabetes. After 72 weeks of tirzepatide treatment, the average weight loss of participants in the 15mg dose group reached as high as 22.5%, which is the first research drug with an average weight loss effect of more than 20% in a phase 3 clinical trial.

On April 17, 2024, Eli Lilly announced that the phase III SURMOUNT-OSA clinical study of tirzepatide for the treatment of moderate to severe obstructive sleep apnea (OSA) combined with obesity reached the main endpoint. At the same time, the company plans to submit a marketing application to the US FDA and other global regulatory agencies soon.

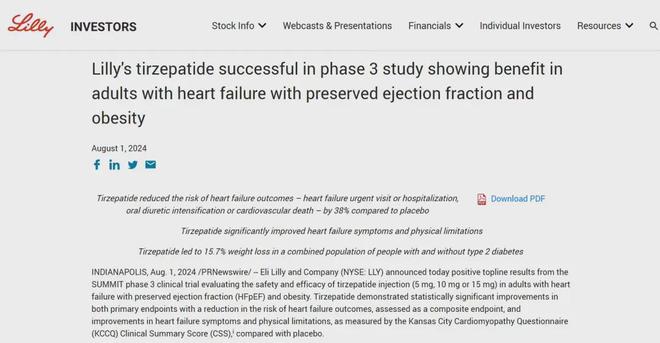
On August 1, 2024, Eli Lilly announced that the phase III SUMMIT study of tirzepatide for the treatment of obesity with heart failure with preserved ejection fraction (HFpEF) achieved positive results. According to the results, the risk of first heart failure in patients in the tirzepatide group was significantly reduced by 38%, and their heart failure symptoms and physical exercise ability were also significantly improved.
On August 27, 2024, Eli Lilly announced that the new prices for the 2.5mg and 5.0mg doses of tirzepatide weight loss version Zepbound were 99.75 US dollars and 137.25 US dollars, respectively, with a price reduction of more than 50%. It is reported that Zepbound has six specifications, namely 2.5/5.0/7.5/10.0/12.5/15.0mg, with target maintenance doses of 5mg/10mg/15mg, depending on treatment response and tolerance.

On October 11, 2024, Eli Lilly announced that it would invest about 1.5 billion yuan to upgrade the production capacity of its Suzhou factory, expanding the production scale of innovative drugs for type 2 diabetes and obesity. It is reported that Eli Lilly Suzhou factory was established in 1996 and currently has more than 3200 employees, covering about 400 cities, with drug research fields covering more than 30 diseases, and the total investment is close to 15 billion yuan.

On October 15, 2024, Eli Lilly announced the establishment of a China Medical Innovation Center in Beijing and planned to set up a Lilly Innovation Incubator to promote clinical research and accelerate drug development. It is reported that the Lilly Innovation Incubator is committed to identifying and supporting high-potential biotechnology enterprises in the early stages of research and development. By integrating Eli Lilly's professional technical resources, LGL will help the next generation of scientific innovation breakthroughs.

On September 15, 2024, Eli Lilly's application for the new indication of tirzepatide injection, "improving the snoring condition of patients with moderate to severe obstructive sleep apnea (OSA) and obesity," was accepted. It is reported that this is the third indication of tirzepatide injection in China after diabetes and weight loss indications.
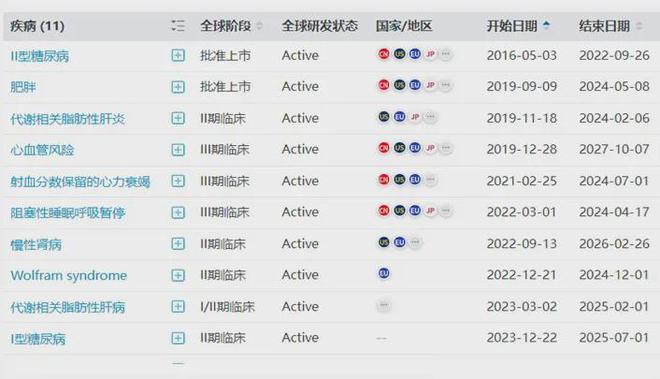
On October 30, 2024, Eli Lilly announced its third-quarter financial report, with a revenue of 31.51 billion US dollars, a year-on-year increase of 27.21%, and a net profit of 6.18 billion US dollars, a year-on-year increase of 102.58%. It is reported that the total sales of tirzepatide in the first three quarters were 11.028 billion US dollars, of which the sales of hypoglycemic version Mounjaro were 8.01 billion US dollars, and the sales of weight loss version Zepbound were 3.018 billion US dollars.
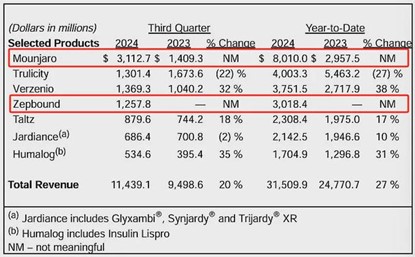
On November 22, 2024, Eli Lilly announced that the new indication application for its self-developed GIP/GLP-1 receptor dual agonist tirzepatide injection was accepted. It is reported that it belongs to chemical drug category 5.1, and the specific indication has not been disclosed. This is the fourth indication applied for by Eli Lilly's tirzepatide in China.
On December 4, 2024, Eli Lilly announced that according to the phase 3b clinical trial data of SURMOUNT-5, at 72 weeks, the weight loss effect of Zepbound, the weight loss version of tirzepatide, was 47% higher than that of Novo Nordisk's weight loss version semaglutide Wegovy. At the same time, Zepbound defeated Wegovy in the main endpoint and all five key secondary endpoints.

On December 5, 2024, Eli Lilly announced that it would invest an additional $3 billion to expand its production base in the United States to increase the production of its diabetes drug Mounjaro and weight loss drug Zepbound. It is reported that the factory in Wisconsin that Eli Lilly is expanding is located in Kenosha County between Chicago and Milwaukee, with a planned total investment of $4 billion. The expanded factory will create 750 high-skilled jobs in the area, including operators, technicians, engineers, and scientists.

On December 20, 2024, Eli Lilly's self-developed GIP/GLP-1 receptor dual agonist tirzepatide injection, Zepbound®, received approval from the US FDA for the indication of "treating obstructive sleep apnea (OSA)." It became the first drug approved to directly treat this common sleep disorder and also marked the second indication approved for Zepbound® in the United States.